Bojdys, M. J.; Briggs, M. E.; Jones, J. T. A.; Adams, D. J.; Chong, S. Y.; Schmidtmann, M.; Cooper,* A. I. Journal of the American Chemical Society 2011, 133, 16566-16571.

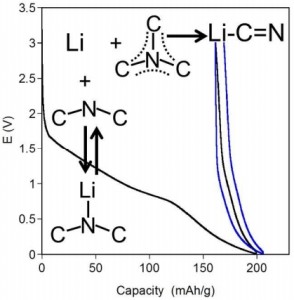
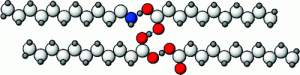
Control over pore size, shape, and connectivity in synthetic porous materials is important in applications such as separation, storage, and catalysis. Crystalline organic cage molecules can exhibit permanent porosity, but there are few synthetic methods to control the crystal packing and hence the pore connectivity. Typically, porosity is either ‘intrinsic’ (within the molecules) or ‘extrinsic’ (between the molecules)—but not both. We report a supramolecular approach to the assembly of porous organic cages which involves bulky directing groups that frustrate the crystal packing. This generates, in a synthetically designed fashion, additional ‘extrinsic’ porosity between the intrinsically porous cage units. One of the molecular crystals exhibits an apparent Brunauer–Emmett–Teller surface area of 854 m2 g–1, which is higher than that of unfunctionalized cages of the same dimensions. Moreover, connectivity between pores, and hence guest uptakes, can be modulated by the introduction of halogen bonding motifs in the cage modules. This suggests a broader approach to the supramolecular engineering of porosity in molecular organic crystals.
DOI: 10.1021/ja2056374 [Download]
Reprinted with permission from Bojdys, M. J.; Briggs, M. E.; Jones, J. T. A.; Adams, D. J.; Chong, S. Y.; Schmidtmann, M.; Cooper, A. I. Journal of the American Chemical Society 2011, 133, 16566-16571. Copyright 2011 American Chemical Society.