Schwarz, D.; Amitava, A.; Ichangi, A.; Kochergin, Y. S.; Lyu, P.; Opanasenko, M. V.; Tarábek, J.; Vacek Chocholoušová, J.; Vacke, J.; Schmidt, J.; Nachtigall, P.; Thomas, A.; Bojdys,* M. J. ChemSusChem 2018. DOI: 10.1002/cssc.201802034 [OPEN ACCESS]
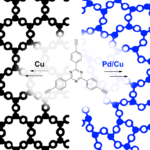 Crystalline and amorphous organic materials are an emergent class of heterogeneous photocatalysts for the generation of hydrogen from water, but a direct correlation between their structures and the resulting properties has not been achieved so far. To make a meaningful comparison between structurally different, yet chemically similar porous polymers, we present two porous polymorphs of a triazine-based graphdiyene (TzG) framework from a simple, one-pot reaction using Cu(I) for TzGCu and Pd(II)/Cu(I) for TzGPd/Cu catalyzed homocoupling polymerization. The polymers form via irreversible coupling reactions and give rise to a crystalline (TzGCu) and an amorphous (TzGPd/Cu) polymorph. Notably, the crystalline and amorphous polymorphs are narrow-gap semiconductors with permanent surface areas of 660 m2 g-1 and 392 m2 g-1, respectively. Hence, both polymers are ideal heterogeneous photocatalysts for water splitting with some of the highest hydrogen evolution rates reported thus far up to 972 μmol h-1 g-1 with and 276 μmol h-1 g-1 without Pt co-catalyst. We conclude, that crystalline order improves delocalisation, while the amorphous polymorph requires a co-catalyst for efficient charge transfer; this will need to be considered in future rational design of polymer catalysts and organic electronics.
Crystalline and amorphous organic materials are an emergent class of heterogeneous photocatalysts for the generation of hydrogen from water, but a direct correlation between their structures and the resulting properties has not been achieved so far. To make a meaningful comparison between structurally different, yet chemically similar porous polymers, we present two porous polymorphs of a triazine-based graphdiyene (TzG) framework from a simple, one-pot reaction using Cu(I) for TzGCu and Pd(II)/Cu(I) for TzGPd/Cu catalyzed homocoupling polymerization. The polymers form via irreversible coupling reactions and give rise to a crystalline (TzGCu) and an amorphous (TzGPd/Cu) polymorph. Notably, the crystalline and amorphous polymorphs are narrow-gap semiconductors with permanent surface areas of 660 m2 g-1 and 392 m2 g-1, respectively. Hence, both polymers are ideal heterogeneous photocatalysts for water splitting with some of the highest hydrogen evolution rates reported thus far up to 972 μmol h-1 g-1 with and 276 μmol h-1 g-1 without Pt co-catalyst. We conclude, that crystalline order improves delocalisation, while the amorphous polymorph requires a co-catalyst for efficient charge transfer; this will need to be considered in future rational design of polymer catalysts and organic electronics.
DOI: 10.1002/cssc.201802034
 Metal-free 2D covalent organic materials transport charges along and in-between π-conjugated layers. Here, we look at the prospects of graphitic carbon nitrides and covalent organic frameworks as 2D semiconductors “beyond graphene and silicon”.
Metal-free 2D covalent organic materials transport charges along and in-between π-conjugated layers. Here, we look at the prospects of graphitic carbon nitrides and covalent organic frameworks as 2D semiconductors “beyond graphene and silicon”.
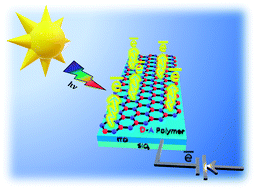 Conventional photoelectrocatalysts composed of precious metals and inorganic elements have limited synthetic design, hence, hampered modularity of their photophysical properties. Here, we demonstrate a scalable, one-pot synthetic approach to grow organic polymer films on the surface of the conventional copper plate under mild conditions. Molecular precursors, containing electron-rich thiophene and electron-deficient triazine-rings, were combined into a donor–acceptor π-conjugated polymer with a broad visible light adsorption range due to a narrow bandgap of 1.42 eV. The strong charge push–pull effect enabled the fabricated donor–acceptor material to have a marked activity as an electrode in a photoelectrochemical cell, reaching anodic photocurrent density of 6.8 μA cm−2 (at 0.6 V vs. Ag/AgCl, pH 7). This value is 3 times higher than that of the model donor–donor thiophene-only-based polymer and twice as high as that of the analogue synthesized in bulk using the heterogenous CuCl catalyst. In addition, the fabricated photoanode showed a 2-fold increase in the photoelectrocatalytic oxygen evolution from water upon simulated sunlight irradiation with the photocurrent density up to 4.8 mA cm−2 (at 1.0 V vs. Ag/AgCl, pH 14). The proposed engineering strategy opens new pathways toward the fabrication of efficient organic “green” materials for photoelectrocatalytic solar energy conversion.
Conventional photoelectrocatalysts composed of precious metals and inorganic elements have limited synthetic design, hence, hampered modularity of their photophysical properties. Here, we demonstrate a scalable, one-pot synthetic approach to grow organic polymer films on the surface of the conventional copper plate under mild conditions. Molecular precursors, containing electron-rich thiophene and electron-deficient triazine-rings, were combined into a donor–acceptor π-conjugated polymer with a broad visible light adsorption range due to a narrow bandgap of 1.42 eV. The strong charge push–pull effect enabled the fabricated donor–acceptor material to have a marked activity as an electrode in a photoelectrochemical cell, reaching anodic photocurrent density of 6.8 μA cm−2 (at 0.6 V vs. Ag/AgCl, pH 7). This value is 3 times higher than that of the model donor–donor thiophene-only-based polymer and twice as high as that of the analogue synthesized in bulk using the heterogenous CuCl catalyst. In addition, the fabricated photoanode showed a 2-fold increase in the photoelectrocatalytic oxygen evolution from water upon simulated sunlight irradiation with the photocurrent density up to 4.8 mA cm−2 (at 1.0 V vs. Ag/AgCl, pH 14). The proposed engineering strategy opens new pathways toward the fabrication of efficient organic “green” materials for photoelectrocatalytic solar energy conversion.
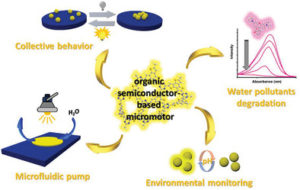 Photosensitive micromotors that can be remotely controlled by visible light irradiation demonstrate great potential in biomedical and environmental applications. To date, a vast number of light‐driven micromotors are mainly composed from costly heavy and precious metal‐containing multicomponent systems, that limit the modularity of chemical and physical properties of these materials. Herein, a highly efficient photocatalytic micromotors based exclusively on a purely organic polymer framework—semiconducting sulfur‐ and nitrogen‐containing donor–acceptor polymer, is presented. Thanks to precisely tuned molecular architecture, this material has the ability to absorb visible light due to a conveniently situated energy gap. In addition, the donor‐acceptor dyads within the polymer backbone ensure efficient photoexcited charge separation. Hence, these polymer‐based micromotors can move in aqueous solutions under visible light illumination via a self‐diffusiophoresis mechanism. Moreover, these micromachines can degrade toxic organic pollutants and respond to an increase in acidity of aqueous environments by instantaneous colour change. The combination of autonomous motility and intrinsic fluorescence enables these organic micromotors to be used as colorimetric and optical sensors for monitoring of the environmental aqueous acidity. The current findings open new pathways toward the design of organic polymer‐based micromotors with tuneable band gap architecture for fabrication of self‐propelled microsensors for environmental control and remediation applications.
Photosensitive micromotors that can be remotely controlled by visible light irradiation demonstrate great potential in biomedical and environmental applications. To date, a vast number of light‐driven micromotors are mainly composed from costly heavy and precious metal‐containing multicomponent systems, that limit the modularity of chemical and physical properties of these materials. Herein, a highly efficient photocatalytic micromotors based exclusively on a purely organic polymer framework—semiconducting sulfur‐ and nitrogen‐containing donor–acceptor polymer, is presented. Thanks to precisely tuned molecular architecture, this material has the ability to absorb visible light due to a conveniently situated energy gap. In addition, the donor‐acceptor dyads within the polymer backbone ensure efficient photoexcited charge separation. Hence, these polymer‐based micromotors can move in aqueous solutions under visible light illumination via a self‐diffusiophoresis mechanism. Moreover, these micromachines can degrade toxic organic pollutants and respond to an increase in acidity of aqueous environments by instantaneous colour change. The combination of autonomous motility and intrinsic fluorescence enables these organic micromotors to be used as colorimetric and optical sensors for monitoring of the environmental aqueous acidity. The current findings open new pathways toward the design of organic polymer‐based micromotors with tuneable band gap architecture for fabrication of self‐propelled microsensors for environmental control and remediation applications.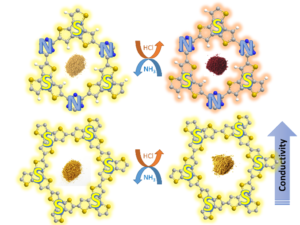 Fully aromatic, organic polymers have the advantage of being composed from light, abundant elements, and are hailed as candidates in electronic and optical devices “beyond silicon”, yet, applications that make use of their π-conjugated backbone and optical bandgap are lacking outside of heterogeneous catalysis. Herein, we use a series of sulfur- and nitrogen-containing porous polymers (SNPs) as real-time optical and electronic sensors reversibly triggered and re-set by acid and ammonia vapors. Our SNPs incorporate donor-acceptor and donor-donor motifs in extended networks and enable us to study the changes in bulk conductivity, optical bandgap, and fluorescence life-times as a function of π-electron de-/localization in the pristine and protonated states. Interestingly, we find that protonated donor-acceptor polymers show a decrease of the optical bandgap by 0.42 eV to 0.76 eV and longer fluorescence life-times. In contrast, protonation of a donor-donor polymer does not affect its bandgap; however, it leads to an increase of electrical conductivity by up to 25-fold and shorter fluorescence life-times. The design strategies highlighted in this study open new avenues towards useful chemical switches and sensors based on modular purely organic materials.
Fully aromatic, organic polymers have the advantage of being composed from light, abundant elements, and are hailed as candidates in electronic and optical devices “beyond silicon”, yet, applications that make use of their π-conjugated backbone and optical bandgap are lacking outside of heterogeneous catalysis. Herein, we use a series of sulfur- and nitrogen-containing porous polymers (SNPs) as real-time optical and electronic sensors reversibly triggered and re-set by acid and ammonia vapors. Our SNPs incorporate donor-acceptor and donor-donor motifs in extended networks and enable us to study the changes in bulk conductivity, optical bandgap, and fluorescence life-times as a function of π-electron de-/localization in the pristine and protonated states. Interestingly, we find that protonated donor-acceptor polymers show a decrease of the optical bandgap by 0.42 eV to 0.76 eV and longer fluorescence life-times. In contrast, protonation of a donor-donor polymer does not affect its bandgap; however, it leads to an increase of electrical conductivity by up to 25-fold and shorter fluorescence life-times. The design strategies highlighted in this study open new avenues towards useful chemical switches and sensors based on modular purely organic materials.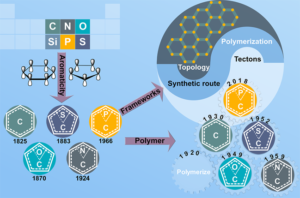 Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.
Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.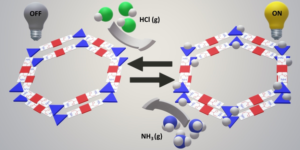
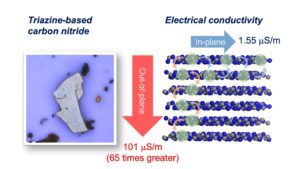 Triazine‐based graphitic carbon nitride (TGCN) is the most recent addition to the family of graphene‐type, two‐dimensional and metal‐free materials. Although hailed as a promising low‐bandgap semiconductor for electronic applications, so far, only its structure and optical properties have been known. Here, we combine direction‐dependent electrical measurements and time‐resolved optical spectroscopy to determine macroscopic conductivity and microscopic charge carrier mobilities in this layered material “beyond graphene”. Electrical conductivity along the basal plane of TGCN is 65‐times lower than through the stacked layers; as opposed to graphite. Furthermore, we develop a model for this charge transport behavior based on observed carrier dynamics and random‐walk simulations. Our combined methods provide a path towards intrinsic charge transport in a direction‐dependent, layered semi‐conductor for applications in field‐effect transistors (FETs) and sensors.
Triazine‐based graphitic carbon nitride (TGCN) is the most recent addition to the family of graphene‐type, two‐dimensional and metal‐free materials. Although hailed as a promising low‐bandgap semiconductor for electronic applications, so far, only its structure and optical properties have been known. Here, we combine direction‐dependent electrical measurements and time‐resolved optical spectroscopy to determine macroscopic conductivity and microscopic charge carrier mobilities in this layered material “beyond graphene”. Electrical conductivity along the basal plane of TGCN is 65‐times lower than through the stacked layers; as opposed to graphite. Furthermore, we develop a model for this charge transport behavior based on observed carrier dynamics and random‐walk simulations. Our combined methods provide a path towards intrinsic charge transport in a direction‐dependent, layered semi‐conductor for applications in field‐effect transistors (FETs) and sensors.