Kulkarni, R.; Huang, J.; Trunk, M.; Burmeister, D.; Amsalem, P.; Müller, J.; Martin, A.; Koch, N.; Kass, D.; Bojdys,* M. J. Chem. Sci. 2021. DOI: 10.1039/D1SC03390E [OPEN ACCESS]
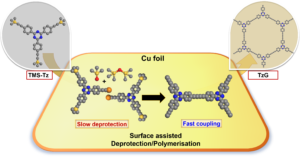 Graphdiyne polymers have interesting electronic properties due to their π-conjugated structure and modular composition. Most of the known synthetic pathways for graphdiyne polymers yield amorphous solids because the irreversible formation of carbon-carbon bonds proceeds under kinetic control and because of defects introduced by the inherent chemical lability of terminal alkyne bonds in the monomers. Here, we present a one-pot surface-assisted deprotection/polymerisation protocol for the synthesis of crystalline graphdiynes over a copper surface starting with stable trimethylsilylated alkyne monomers. In comparison to conventional polymerisation protocols, our method yields large-area crystalline thin graphdiyne films and, at the same time, minimises detrimental effects on the monomers like oxidation or cyclotrimerisation side reactions typically associated with terminal alkynes. A detailed study of the reaction mechanism reveals that the deprotection and polymerisation of the monomer is promoted by Cu(II) oxide/hydroxide species on the as-received copper surface. These findings pave the way for the scalable synthesis of crystalline graphdiyne-based materials as cohesive thin films.
Graphdiyne polymers have interesting electronic properties due to their π-conjugated structure and modular composition. Most of the known synthetic pathways for graphdiyne polymers yield amorphous solids because the irreversible formation of carbon-carbon bonds proceeds under kinetic control and because of defects introduced by the inherent chemical lability of terminal alkyne bonds in the monomers. Here, we present a one-pot surface-assisted deprotection/polymerisation protocol for the synthesis of crystalline graphdiynes over a copper surface starting with stable trimethylsilylated alkyne monomers. In comparison to conventional polymerisation protocols, our method yields large-area crystalline thin graphdiyne films and, at the same time, minimises detrimental effects on the monomers like oxidation or cyclotrimerisation side reactions typically associated with terminal alkynes. A detailed study of the reaction mechanism reveals that the deprotection and polymerisation of the monomer is promoted by Cu(II) oxide/hydroxide species on the as-received copper surface. These findings pave the way for the scalable synthesis of crystalline graphdiyne-based materials as cohesive thin films.
