Schwarz, D.; Amitava, A.; Ichangi, A.; Kochergin, Y. S.; Lyu, P.; Opanasenko, M. V.; Tarábek, J.; Vacek Chocholoušová, J.; Vacke, J.; Schmidt, J.; Nachtigall, P.; Thomas, A.; Bojdys,* M. J. ChemSusChem 2018. DOI: 10.1002/cssc.201802034 [OPEN ACCESS]
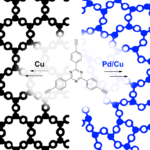 Crystalline and amorphous organic materials are an emergent class of heterogeneous photocatalysts for the generation of hydrogen from water, but a direct correlation between their structures and the resulting properties has not been achieved so far. To make a meaningful comparison between structurally different, yet chemically similar porous polymers, we present two porous polymorphs of a triazine-based graphdiyene (TzG) framework from a simple, one-pot reaction using Cu(I) for TzGCu and Pd(II)/Cu(I) for TzGPd/Cu catalyzed homocoupling polymerization. The polymers form via irreversible coupling reactions and give rise to a crystalline (TzGCu) and an amorphous (TzGPd/Cu) polymorph. Notably, the crystalline and amorphous polymorphs are narrow-gap semiconductors with permanent surface areas of 660 m2 g-1 and 392 m2 g-1, respectively. Hence, both polymers are ideal heterogeneous photocatalysts for water splitting with some of the highest hydrogen evolution rates reported thus far up to 972 μmol h-1 g-1 with and 276 μmol h-1 g-1 without Pt co-catalyst. We conclude, that crystalline order improves delocalisation, while the amorphous polymorph requires a co-catalyst for efficient charge transfer; this will need to be considered in future rational design of polymer catalysts and organic electronics.
Crystalline and amorphous organic materials are an emergent class of heterogeneous photocatalysts for the generation of hydrogen from water, but a direct correlation between their structures and the resulting properties has not been achieved so far. To make a meaningful comparison between structurally different, yet chemically similar porous polymers, we present two porous polymorphs of a triazine-based graphdiyene (TzG) framework from a simple, one-pot reaction using Cu(I) for TzGCu and Pd(II)/Cu(I) for TzGPd/Cu catalyzed homocoupling polymerization. The polymers form via irreversible coupling reactions and give rise to a crystalline (TzGCu) and an amorphous (TzGPd/Cu) polymorph. Notably, the crystalline and amorphous polymorphs are narrow-gap semiconductors with permanent surface areas of 660 m2 g-1 and 392 m2 g-1, respectively. Hence, both polymers are ideal heterogeneous photocatalysts for water splitting with some of the highest hydrogen evolution rates reported thus far up to 972 μmol h-1 g-1 with and 276 μmol h-1 g-1 without Pt co-catalyst. We conclude, that crystalline order improves delocalisation, while the amorphous polymorph requires a co-catalyst for efficient charge transfer; this will need to be considered in future rational design of polymer catalysts and organic electronics.
