Roeser,* J.; Dragica, P.; Bojdys, M. J.; Fayon, P.; Trewin, A.; Fitch, A. N.; Schmidt, M. U.; Thomas,* A. Nature Chemistry 2017. DOI: 10.1038/NCHEM.2771
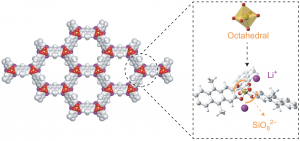
Crystalline frameworks composed of hexacoordinated silicon species have thus far only been observed in a few high pressure silicate phases. By implementing reversible Si–O chemistry for the crystallization of covalent organic frameworks, we demonstrate the simple one-pot synthesis of silicate organic frameworks based on octahedral dianionic SiO6 building units. Clear evidence of the hexacoordinated environment around the silicon atoms is given by 29Si nuclear magnetic resonance analysis. Characterization by high-resolution powder X-ray diffraction, density functional theory calculation and analysis of the pair-distribution function showed that those anionic frameworks—M2[Si(C16H10O4)1.5], where M = Li, Na, K and C16H10O4 is 9,10-dimethyl-2,3,6,7-tetraolatoanthracene—crystallize as two-dimensional hexagonal layers stabilized in a fully eclipsed stacking arrangement with pronounced disorder in the stacking direction. Permanent microporosity with a two-step filling process was evidenced by gas-sorption measurements. The negatively charged backbone balanced with extra-framework cations and the permanent microporosity are characteristics that are shared with zeolites.
