Burmeister, D.; Tran, H. A.; Müller, J.; Guerrini, M.; Cocchi, C.; Plaickner J.; Kochovski, Z.: List-Kratochvil, E.; Bojdys,* M. J. Angew. Chem. Int. Ed. 2021. DOI: 10.1002/anie.202111749 [OPEN ACCESS]
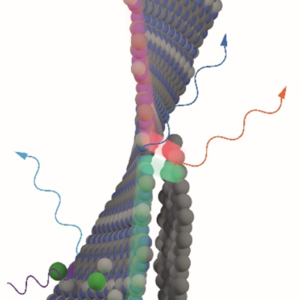 Crystalline semiconducting carbon nitrides are chemically and physically resilient, consist of earth abundant elements, and can be exfoliated into 2D atomically thin layers. In particular, poly(triazine imide) (PTI) is a highly crystalline semiconductor, and though no techniques exist to date that enable synthesis of macroscopic monolayers of PTI, it is possible to study it in thin layer device applications that are compatible with its polycrystalline, nanoscale morphology. In our study, we find that the by-product of conventional PTI synthesis is a C-C carbon rich phase that is detrimental for charge transport and photoluminescence. An optimised synthetic protocol yields a PTI material with an increased quantum yield, enabled photocurrent and electroluminescence. In addition, we report that protonation of the PTI structure happens preferentially at the pyridinic nitrogen atoms of the triazine (C3N3) rings, is accompanied by exfoliation of PTI layers, and contributes to increases in quantum yield and exciton lifetimes. This study describes structure-property relationships in PTI that link (i) the nature of defects, their formation, and how to avoid them with (ii) the optical and electronic performance of PTI. On the basis of our findings, we create an OLED prototype with PTI as the active, metal-free material, and we lay the foundations for device integration of solution-processable graphitic carbon nitride dispersions in semiconductor devices.
Crystalline semiconducting carbon nitrides are chemically and physically resilient, consist of earth abundant elements, and can be exfoliated into 2D atomically thin layers. In particular, poly(triazine imide) (PTI) is a highly crystalline semiconductor, and though no techniques exist to date that enable synthesis of macroscopic monolayers of PTI, it is possible to study it in thin layer device applications that are compatible with its polycrystalline, nanoscale morphology. In our study, we find that the by-product of conventional PTI synthesis is a C-C carbon rich phase that is detrimental for charge transport and photoluminescence. An optimised synthetic protocol yields a PTI material with an increased quantum yield, enabled photocurrent and electroluminescence. In addition, we report that protonation of the PTI structure happens preferentially at the pyridinic nitrogen atoms of the triazine (C3N3) rings, is accompanied by exfoliation of PTI layers, and contributes to increases in quantum yield and exciton lifetimes. This study describes structure-property relationships in PTI that link (i) the nature of defects, their formation, and how to avoid them with (ii) the optical and electronic performance of PTI. On the basis of our findings, we create an OLED prototype with PTI as the active, metal-free material, and we lay the foundations for device integration of solution-processable graphitic carbon nitride dispersions in semiconductor devices.
Press-releases: [IRIS, EN], [IRIS, DE], [KCL, EN], [HU, DE]
DOI: 10.1002/anie.202111749
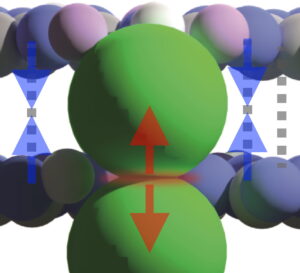 Ionothermal condensation of dicyandiamide in alkali halide salt melts leads to the formation of extended 2D, layered frameworks only in the presence of small halides such as chloride, and bromide. With increasing size of the alkali halide intercalate, stabilizing van der Waals interactions between extended, π-conjugated triazine-based sheets are lost. We identify the main, crystalline product from an alkali iodide eutectic as melem hydrate, a heptazine (C6N7)-based, hydrogen-bonded, monoclinic solid.
Ionothermal condensation of dicyandiamide in alkali halide salt melts leads to the formation of extended 2D, layered frameworks only in the presence of small halides such as chloride, and bromide. With increasing size of the alkali halide intercalate, stabilizing van der Waals interactions between extended, π-conjugated triazine-based sheets are lost. We identify the main, crystalline product from an alkali iodide eutectic as melem hydrate, a heptazine (C6N7)-based, hydrogen-bonded, monoclinic solid.

 Crystalline semiconducting carbon nitrides are chemically and physically resilient, consist of earth abundant elements, and can be exfoliated into 2D atomically thin layers. In particular, poly(triazine imide) (PTI) is a highly crystalline semiconductor, and though no techniques exist to date that enable synthesis of macroscopic monolayers of PTI, it is possible to study it in thin layer device applications that are compatible with its polycrystalline, nanoscale morphology. In our study, we find that the by-product of conventional PTI synthesis is a C-C carbon rich phase that is detrimental for charge transport and photoluminescence. An optimised synthetic protocol yields a PTI material with an increased quantum yield, enabled photocurrent and electroluminescence. In addition, we report that protonation of the PTI structure happens preferentially at the pyridinic nitrogen atoms of the triazine (C3N3) rings, is accompanied by exfoliation of PTI layers, and contributes to increases in quantum yield and exciton lifetimes. This study describes structure-property relationships in PTI that link (i) the nature of defects, their formation, and how to avoid them with (ii) the optical and electronic performance of PTI. On the basis of our findings, we create an OLED prototype with PTI as the active, metal-free material, and we lay the foundations for device integration of solution-processable graphitic carbon nitride dispersions in semiconductor devices.
Crystalline semiconducting carbon nitrides are chemically and physically resilient, consist of earth abundant elements, and can be exfoliated into 2D atomically thin layers. In particular, poly(triazine imide) (PTI) is a highly crystalline semiconductor, and though no techniques exist to date that enable synthesis of macroscopic monolayers of PTI, it is possible to study it in thin layer device applications that are compatible with its polycrystalline, nanoscale morphology. In our study, we find that the by-product of conventional PTI synthesis is a C-C carbon rich phase that is detrimental for charge transport and photoluminescence. An optimised synthetic protocol yields a PTI material with an increased quantum yield, enabled photocurrent and electroluminescence. In addition, we report that protonation of the PTI structure happens preferentially at the pyridinic nitrogen atoms of the triazine (C3N3) rings, is accompanied by exfoliation of PTI layers, and contributes to increases in quantum yield and exciton lifetimes. This study describes structure-property relationships in PTI that link (i) the nature of defects, their formation, and how to avoid them with (ii) the optical and electronic performance of PTI. On the basis of our findings, we create an OLED prototype with PTI as the active, metal-free material, and we lay the foundations for device integration of solution-processable graphitic carbon nitride dispersions in semiconductor devices.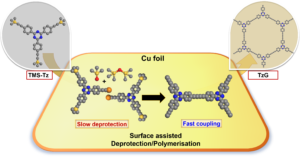 Graphdiyne polymers have interesting electronic properties due to their π-conjugated structure and modular composition. Most of the known synthetic pathways for graphdiyne polymers yield amorphous solids because the irreversible formation of carbon-carbon bonds proceeds under kinetic control and because of defects introduced by the inherent chemical lability of terminal alkyne bonds in the monomers. Here, we present a one-pot surface-assisted deprotection/polymerisation protocol for the synthesis of crystalline graphdiynes over a copper surface starting with stable trimethylsilylated alkyne monomers. In comparison to conventional polymerisation protocols, our method yields large-area crystalline thin graphdiyne films and, at the same time, minimises detrimental effects on the monomers like oxidation or cyclotrimerisation side reactions typically associated with terminal alkynes. A detailed study of the reaction mechanism reveals that the deprotection and polymerisation of the monomer is promoted by Cu(II) oxide/hydroxide species on the as-received copper surface. These findings pave the way for the scalable synthesis of crystalline graphdiyne-based materials as cohesive thin films.
Graphdiyne polymers have interesting electronic properties due to their π-conjugated structure and modular composition. Most of the known synthetic pathways for graphdiyne polymers yield amorphous solids because the irreversible formation of carbon-carbon bonds proceeds under kinetic control and because of defects introduced by the inherent chemical lability of terminal alkyne bonds in the monomers. Here, we present a one-pot surface-assisted deprotection/polymerisation protocol for the synthesis of crystalline graphdiynes over a copper surface starting with stable trimethylsilylated alkyne monomers. In comparison to conventional polymerisation protocols, our method yields large-area crystalline thin graphdiyne films and, at the same time, minimises detrimental effects on the monomers like oxidation or cyclotrimerisation side reactions typically associated with terminal alkynes. A detailed study of the reaction mechanism reveals that the deprotection and polymerisation of the monomer is promoted by Cu(II) oxide/hydroxide species on the as-received copper surface. These findings pave the way for the scalable synthesis of crystalline graphdiyne-based materials as cohesive thin films.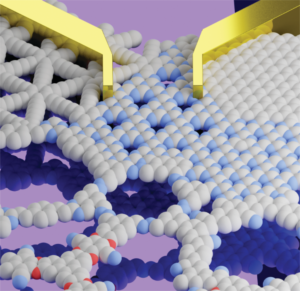 Metal-free 2D covalent organic materials transport charges along and in-between π-conjugated layers. Here, we look at the prospects of graphitic carbon nitrides and covalent organic frameworks as 2D semiconductors “beyond graphene and silicon”.
Metal-free 2D covalent organic materials transport charges along and in-between π-conjugated layers. Here, we look at the prospects of graphitic carbon nitrides and covalent organic frameworks as 2D semiconductors “beyond graphene and silicon”.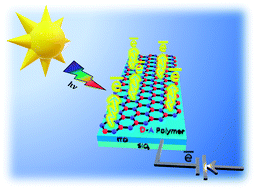 Conventional photoelectrocatalysts composed of precious metals and inorganic elements have limited synthetic design, hence, hampered modularity of their photophysical properties. Here, we demonstrate a scalable, one-pot synthetic approach to grow organic polymer films on the surface of the conventional copper plate under mild conditions. Molecular precursors, containing electron-rich thiophene and electron-deficient triazine-rings, were combined into a donor–acceptor π-conjugated polymer with a broad visible light adsorption range due to a narrow bandgap of 1.42 eV. The strong charge push–pull effect enabled the fabricated donor–acceptor material to have a marked activity as an electrode in a photoelectrochemical cell, reaching anodic photocurrent density of 6.8 μA cm−2 (at 0.6 V vs. Ag/AgCl, pH 7). This value is 3 times higher than that of the model donor–donor thiophene-only-based polymer and twice as high as that of the analogue synthesized in bulk using the heterogenous CuCl catalyst. In addition, the fabricated photoanode showed a 2-fold increase in the photoelectrocatalytic oxygen evolution from water upon simulated sunlight irradiation with the photocurrent density up to 4.8 mA cm−2 (at 1.0 V vs. Ag/AgCl, pH 14). The proposed engineering strategy opens new pathways toward the fabrication of efficient organic “green” materials for photoelectrocatalytic solar energy conversion.
Conventional photoelectrocatalysts composed of precious metals and inorganic elements have limited synthetic design, hence, hampered modularity of their photophysical properties. Here, we demonstrate a scalable, one-pot synthetic approach to grow organic polymer films on the surface of the conventional copper plate under mild conditions. Molecular precursors, containing electron-rich thiophene and electron-deficient triazine-rings, were combined into a donor–acceptor π-conjugated polymer with a broad visible light adsorption range due to a narrow bandgap of 1.42 eV. The strong charge push–pull effect enabled the fabricated donor–acceptor material to have a marked activity as an electrode in a photoelectrochemical cell, reaching anodic photocurrent density of 6.8 μA cm−2 (at 0.6 V vs. Ag/AgCl, pH 7). This value is 3 times higher than that of the model donor–donor thiophene-only-based polymer and twice as high as that of the analogue synthesized in bulk using the heterogenous CuCl catalyst. In addition, the fabricated photoanode showed a 2-fold increase in the photoelectrocatalytic oxygen evolution from water upon simulated sunlight irradiation with the photocurrent density up to 4.8 mA cm−2 (at 1.0 V vs. Ag/AgCl, pH 14). The proposed engineering strategy opens new pathways toward the fabrication of efficient organic “green” materials for photoelectrocatalytic solar energy conversion.
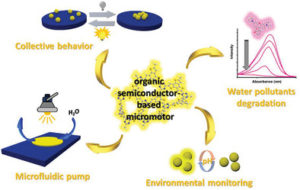 Photosensitive micromotors that can be remotely controlled by visible light irradiation demonstrate great potential in biomedical and environmental applications. To date, a vast number of light‐driven micromotors are mainly composed from costly heavy and precious metal‐containing multicomponent systems, that limit the modularity of chemical and physical properties of these materials. Herein, a highly efficient photocatalytic micromotors based exclusively on a purely organic polymer framework—semiconducting sulfur‐ and nitrogen‐containing donor–acceptor polymer, is presented. Thanks to precisely tuned molecular architecture, this material has the ability to absorb visible light due to a conveniently situated energy gap. In addition, the donor‐acceptor dyads within the polymer backbone ensure efficient photoexcited charge separation. Hence, these polymer‐based micromotors can move in aqueous solutions under visible light illumination via a self‐diffusiophoresis mechanism. Moreover, these micromachines can degrade toxic organic pollutants and respond to an increase in acidity of aqueous environments by instantaneous colour change. The combination of autonomous motility and intrinsic fluorescence enables these organic micromotors to be used as colorimetric and optical sensors for monitoring of the environmental aqueous acidity. The current findings open new pathways toward the design of organic polymer‐based micromotors with tuneable band gap architecture for fabrication of self‐propelled microsensors for environmental control and remediation applications.
Photosensitive micromotors that can be remotely controlled by visible light irradiation demonstrate great potential in biomedical and environmental applications. To date, a vast number of light‐driven micromotors are mainly composed from costly heavy and precious metal‐containing multicomponent systems, that limit the modularity of chemical and physical properties of these materials. Herein, a highly efficient photocatalytic micromotors based exclusively on a purely organic polymer framework—semiconducting sulfur‐ and nitrogen‐containing donor–acceptor polymer, is presented. Thanks to precisely tuned molecular architecture, this material has the ability to absorb visible light due to a conveniently situated energy gap. In addition, the donor‐acceptor dyads within the polymer backbone ensure efficient photoexcited charge separation. Hence, these polymer‐based micromotors can move in aqueous solutions under visible light illumination via a self‐diffusiophoresis mechanism. Moreover, these micromachines can degrade toxic organic pollutants and respond to an increase in acidity of aqueous environments by instantaneous colour change. The combination of autonomous motility and intrinsic fluorescence enables these organic micromotors to be used as colorimetric and optical sensors for monitoring of the environmental aqueous acidity. The current findings open new pathways toward the design of organic polymer‐based micromotors with tuneable band gap architecture for fabrication of self‐propelled microsensors for environmental control and remediation applications.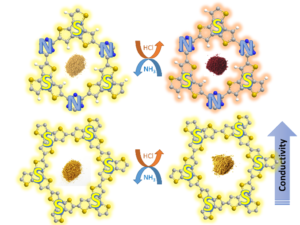 Fully aromatic, organic polymers have the advantage of being composed from light, abundant elements, and are hailed as candidates in electronic and optical devices “beyond silicon”, yet, applications that make use of their π-conjugated backbone and optical bandgap are lacking outside of heterogeneous catalysis. Herein, we use a series of sulfur- and nitrogen-containing porous polymers (SNPs) as real-time optical and electronic sensors reversibly triggered and re-set by acid and ammonia vapors. Our SNPs incorporate donor-acceptor and donor-donor motifs in extended networks and enable us to study the changes in bulk conductivity, optical bandgap, and fluorescence life-times as a function of π-electron de-/localization in the pristine and protonated states. Interestingly, we find that protonated donor-acceptor polymers show a decrease of the optical bandgap by 0.42 eV to 0.76 eV and longer fluorescence life-times. In contrast, protonation of a donor-donor polymer does not affect its bandgap; however, it leads to an increase of electrical conductivity by up to 25-fold and shorter fluorescence life-times. The design strategies highlighted in this study open new avenues towards useful chemical switches and sensors based on modular purely organic materials.
Fully aromatic, organic polymers have the advantage of being composed from light, abundant elements, and are hailed as candidates in electronic and optical devices “beyond silicon”, yet, applications that make use of their π-conjugated backbone and optical bandgap are lacking outside of heterogeneous catalysis. Herein, we use a series of sulfur- and nitrogen-containing porous polymers (SNPs) as real-time optical and electronic sensors reversibly triggered and re-set by acid and ammonia vapors. Our SNPs incorporate donor-acceptor and donor-donor motifs in extended networks and enable us to study the changes in bulk conductivity, optical bandgap, and fluorescence life-times as a function of π-electron de-/localization in the pristine and protonated states. Interestingly, we find that protonated donor-acceptor polymers show a decrease of the optical bandgap by 0.42 eV to 0.76 eV and longer fluorescence life-times. In contrast, protonation of a donor-donor polymer does not affect its bandgap; however, it leads to an increase of electrical conductivity by up to 25-fold and shorter fluorescence life-times. The design strategies highlighted in this study open new avenues towards useful chemical switches and sensors based on modular purely organic materials.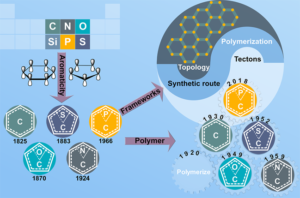 Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.
Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.