Takashi Nakanishi (Editor)
Chapter 7. Polymeric Frameworks: Toward Porous Semiconductors
Weber, J.; Bojdys, M. J.; Thomas, A.
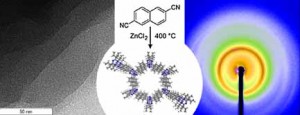
In this chapter, we describe pathways for the preparation of porous, polymeric semiconductors. Several examples that show high prospects for applications such as organic solar cells, organic light-emitting diodes (OLEDs) and organic field effect transistors (OFETs) have been reported and will be the subject of discussion. In the first section, an overview of the general methods of preparation for meso- and microporous polymers is presented. Several examples are shown illustrating the different synthetic strategies. The second section focuses on porous networks with pore walls composed of conjugated, semiconducting polymers. Here, the relationship between the development of a 3D organic semiconductor and porous semiconducting polymers will be discussed. The section also gives a comprehensive overview over known examples of porous semiconducting polymers which have not only been put to work in optoelectronic devices, but have also been successfully used as materials for gas storage or as catalyst support. The third section describes a particular type of porous conjugated polymers, namely, covalent organic frameworks (COFs). The last section discusses the organic semiconductor, graphitic carbon nitride. The semiconducting properties of this material have been recently extensively investigated and used for important applications, such as photocatalysis for the production of hydrogen from water.