Huang, J.; Tarábek, J.; Kulkarni, R.; Wang, C.; Dračínský, M.; Smales, G. J.; Tian, Y.; Ren, S.; Pauw, B. R.; Resch-Genger, U.; Bojdys,* M. J. Chem. Eur. J. 2019. DOI: 10.1002/chem.201900281 [OPEN ACCESS]
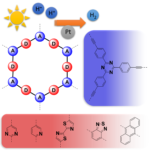
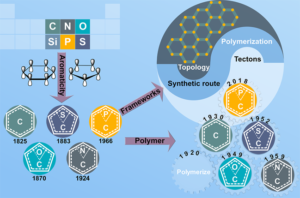 Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.
Structural modularity of polymer frameworks is a key advantage of covalent organic polymers, however, only C, N, O, Si and S have found their way into their building blocks so far. Here, we expand the toolbox available to polymer and materials chemists by one additional nonmetal, phosphorus. Starting with a building block that contains a λ5‐phosphinine (C5P) moiety, we evaluate a number of polymerisation protocols, finally obtaining a π‐conjugated, covalent phosphinine‐based framework (CPF‐1) via Suzuki‐Miyaura coupling. CPF‐1 is a weakly porous polymer glass (72.4 m2 g-1 N2 BET at 77 K) with green fluorescence (λmax 546 nm) and extremely high thermal stability. The polymer catalyzes hydrogen evolution from water under UV and visible light irradiation without the need for additional co‐catalyst at a rate of 33.3 μmol h-1 g-1. Our results demonstrate for the first time the incorporation of the phosphinine motif into a complex polymer framework. Phosphinine‐based frameworks show promising electronic and optical properties that might spark future interest in their applications in light‐emitting devices and heterogeneous catalysis.


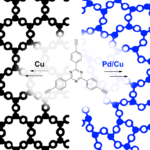
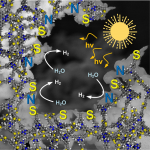
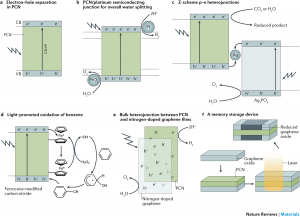
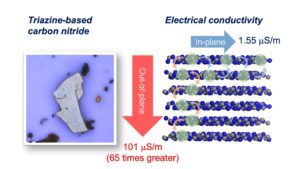 Triazine‐based graphitic carbon nitride (TGCN) is the most recent addition to the family of graphene‐type, two‐dimensional and metal‐free materials. Although hailed as a promising low‐bandgap semiconductor for electronic applications, so far, only its structure and optical properties have been known. Here, we combine direction‐dependent electrical measurements and time‐resolved optical spectroscopy to determine macroscopic conductivity and microscopic charge carrier mobilities in this layered material “beyond graphene”. Electrical conductivity along the basal plane of TGCN is 65‐times lower than through the stacked layers; as opposed to graphite. Furthermore, we develop a model for this charge transport behavior based on observed carrier dynamics and random‐walk simulations. Our combined methods provide a path towards intrinsic charge transport in a direction‐dependent, layered semi‐conductor for applications in field‐effect transistors (FETs) and sensors.
Triazine‐based graphitic carbon nitride (TGCN) is the most recent addition to the family of graphene‐type, two‐dimensional and metal‐free materials. Although hailed as a promising low‐bandgap semiconductor for electronic applications, so far, only its structure and optical properties have been known. Here, we combine direction‐dependent electrical measurements and time‐resolved optical spectroscopy to determine macroscopic conductivity and microscopic charge carrier mobilities in this layered material “beyond graphene”. Electrical conductivity along the basal plane of TGCN is 65‐times lower than through the stacked layers; as opposed to graphite. Furthermore, we develop a model for this charge transport behavior based on observed carrier dynamics and random‐walk simulations. Our combined methods provide a path towards intrinsic charge transport in a direction‐dependent, layered semi‐conductor for applications in field‐effect transistors (FETs) and sensors.